Main areas of interest:
- Neuromodulatory actions on PPN neurons and pathophysiological actions modifying them
- Role of astrocyte-neuron communication in the regulation of neuronal excitability
The pedunculopontine nucleus (PPN) is a cholinergic nucleus of the reticular activating system, which provides cholinergic input to several subcortical structures. The PPN plays a significant role in sensory gating, regulation of sleep-wakefulness cycles and movement. It also has pathophysiological significance as the PPN is affected in certain types of Parkinson's disease, progressive supranuclear palsy, schizophrenia and peduncular hallucinosis.
PPN neurons are regulated by several, partially overlapping neuromodulatory actions. We demonstrated that several neuromodulatory actions (endocannabinoid, cholinergic, serotonergic) induce astrocyte- and metabotropic glutamate receptor dependent neuronal depolarization or hyperpolarization. Together with tonic effects, phasic, astrocyte- and extrasynaptic NMDA receptor dependent excitatory currents (slow inward currents, SICs) were also observed.
Although ex vivo experiments revealed robust actions, their in vivo significance has not been described yet. We aim to reveal the physiological and pathophysiological significance of astrocyte (over)activation on neuromodulatory actions targeting the PPN. For this, the combination of chemo- and optogenetic astrocyte activation and behavioural tests are used.
The SICs are characteristic features of astrocyte-neuron communication. This phenomenon is investigated by using murine and human neocortical samples. Our aim is to demonstrate the role of SICs in synaptic plasticity and its age-dependence. Based on our data, this aspect of astrocyte-neuron communication is differentially affected by normal aging in mice and humans.
Techniques:
- Maintenance and usage of transgenic mouse strains (strains expressing fluorescent markers, opto- and chemogenetic actuators, glutamate- and calcium sensors)
- Combined slice electrophysiology and calcium imaging
- Optogenetics, chemogenetics, flash photolysis
- Morphological reconstruction of neurons and astrocytes, immunohistochemistry
- Experiments on human acute brain slices on samples from surgery
- Behavioural tests
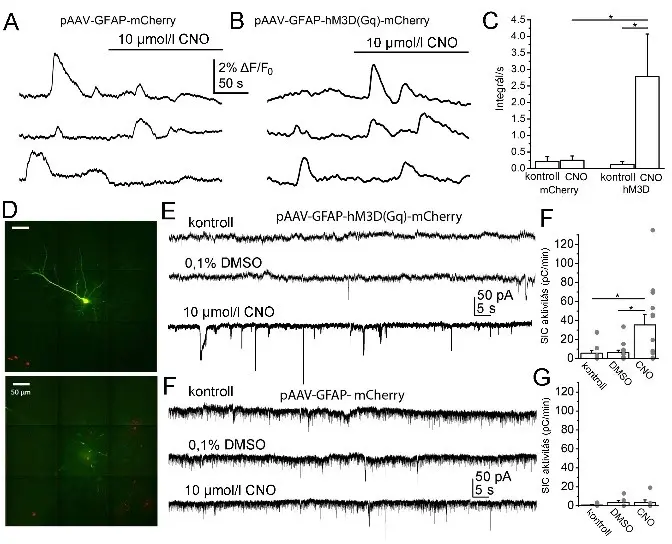
Fig. 1.
The chemogenetic activation of astrocytes increases Ca2+-wave activity of astrocytes and SIC activity on neurons. A.
epresentative calcium wave traces from samples expressing mCherry tag under GFAP promoter from 3 individual regions of interest. B. Representative calcium wave traces from samples expressing hM3D(Gq) chemogenetic actuator under GFAP promoter from 3 individual recordings. C. Statistical summary of changes in calcium wave areas from different samples under different conditions. D. A neocortical pyramidal cell labelled with biocytin during the patch clamp experiment (green) and astrocytes expressing mCherry tag (red) on two confocal z-stack images (scale bar: 50 µm). E. Representative traces under control conditions, with 0.1% DMSO and with 10 µM CNO from a sample expressing hM3D(Gq) chemogenetic actuator under GFAP promoter. F. Statistical comparison of SIC activity under the conditions shown on panel E (hollow columns: average ± SEM, grey dots: individual data). G. Representative traces recorded in control (black), with DMSO (blue), and 10 µM CNO (red) from a sample lacking hM3D(Gq) chemogenetic actuator but expressing only mCherry tag under GFAP promoter. H. Statistical comparison of SIC activity under conditions shown on panel H (hollow columns: average ± SEM, grey dots: individual data). (*: p<0.05; **: p<0.01; ***: p<0.001)
Figure 2
Acoustic startle measurements. a-e. Components of the startle setup: (a) force measuring setup and the polystyrene box containing (b) the siren, (c) the loudspeakers, (d) the web camera and (e) the experimental animal. (f) 5 representative concomitant startle reactions and their statistical evaluation (normalized to the first amplitude; average ± SEM; dashed line: 1).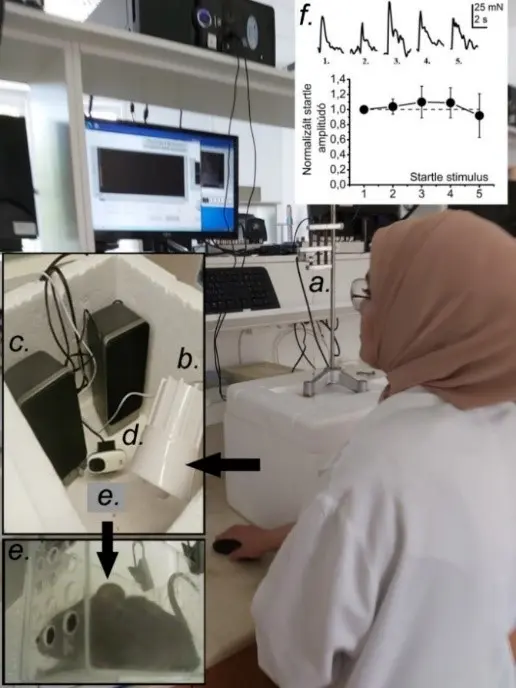
Lab members:
- Dr. Balázs Pál (associate professor)
- Dr. Krisztina Deák-Pocsai (research fellow, part-time)
- Dr. Adrienn Kovács (junior research fellow)
- Baneen Maamrah (PhD student, Stipendium Hungaricum)
- Andrea Csemer (junior research fellow, PhD student)
- Cintia Sokvári (PhD student)
Alumni:
- Csilla Bordás (PhD student, graduation: 2016)
- Ágnes Kovács (PhD student)
- Dr. Brigitta Baksa (PhD student, graduation: 2020)
- Dr. Tsogbadrakh Bayasgalan (PhD student, Stipendium Hungaricum, graduation: 2022. At present, he is postdoctoral fellow in the laboratory of Dr. Juan Mena-Segovia, Rutgers University, NJ, USA)
External collaborations:
- Dr. Imre Kalló (Institute of Experimental Medicine, Budapest, Hungary)
- Dr. Juan Mena-Segovia (Rutgers University, NJ, USA)
- Dr. Guillermo Spitzmaul (Universidad Nacional del Sur, Bahia Blanca, Argentina)
Selected publications:
Kőszeghy Á, Kovács A, Bíró T, Szücs P, Vincze J, Hegyi Z, Antal M, Pál B (2015) Endocannabinoid signaling modulates neurons of the pedunculopontine nucleus (PPN) via actions on the neuron-glia communication. Brain Structure and Function, 220(5):3023-41.
Kovács A, Bordás Cs, Bíró T, Hegyi Z, Antal M, Szücs P, Pál B (2017) Direct presynaptic and indirect astrocyte-mediated mechanisms both contribute to endocannabinoid signaling in the pedunculopontine nucleus of mice. Brain Structure and Function, 222(1): 247-266. D1 (Anatomy, medicine)
Kovacs A, Pál B (2017) Astrocyte-dependent slow inward currents (SICs) participate in neuromodulatory mechanisms in the pedunculopontine nucleus (PPN). Front Cell Neurosci.; 11:16. Q1
Bardóczi Z, Pál B, Kőszeghy Á, Wilheim T, Watanabe M, Záborszky L, Liposits Z, Kalló I (2017) Glycinergic input to the mouse basal forebrain cholinergic neurons. J Neurosci. 37(39):9534-9549. D1
Pál B (2018) Involvement of extrasynaptic glutamate in physiological and pathophysiological changes of neuronal excitability. Cellular and Molecular Life Sciences, 75(16): 2917-2949. D1
Baksa B, Kovács A, Bayasgalan T, Szentesi P, Kőszeghy Á, Szücs P, Pál B. (2019) Characterization of functional subgroups among genetically identified cholinergic neurons in the pedunculopontine nucleus. Cellular and Molecular Life Sciences, 76(14):2799-2815, D1
Dautan D, Kovács A, Bayasgalan T, Diaz-Acevedo MA, Pal B, Mena-Segovia J. (2021) Modulation of motor behavior by the mesencephalic locomotor region. Cell Rep. 36(8):109594. D1
Bayasgalan T, Stupniki S, Kovács A, Csemer A, Szentesi P, Pocsai K, Dionisio L, Spitzmaul G, Pál B. (2021) Alteration of mesopontine cholinergic function by the lack of KCNQ4 subunit. Front Cell Neurosci. 15:707789. Q1