We are examining the expression and function of Septins (which are accepted as the forth component of the cytoskeleton) in skeletal muscle and myogenic cell cultures. In this project our goal is to answer the following questions:
- Does Septin 7 participate in skeletal muscle regeneration in control (BL6/C57) mice?
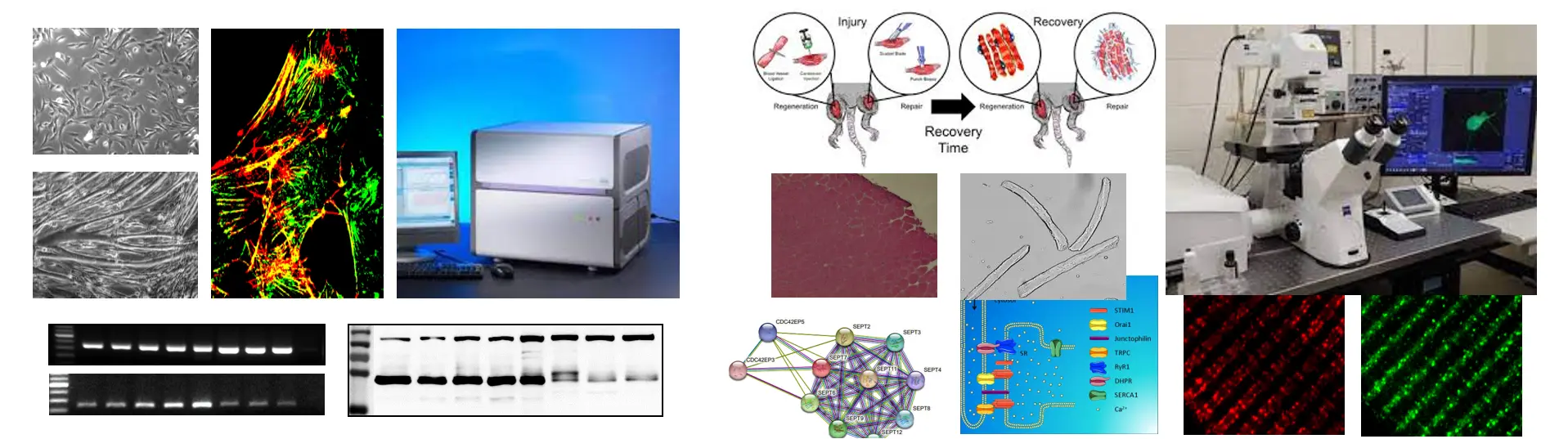
Skeletal muscle structure is restored after injury by a process called regeneration. Initial inflammatory response is followed by regeneration and completed by maturation and remodelilng of newly formed myofibers. Muscle stem cells (MuSCs), also called satellite cells, are localized between basal lamina and muscle fibers in the stem cell niche. During regeneration MuSCs are activated, committed myogenic progenitor cells turn into myoblasts, and they organize into myotubes and fuse with the injured fiber It is followed by the formation of new myofibers and completed by the restoration of skeletal muscle structure. Expression level of PAX7 – the biomarker of skeletal muscle regeneration - shows strong correlation to phases of regeneration. Recently published studies indicate important role of septins in the arrangement of both cytoskeletal proteins and proteins involved in intracellular calcium signaling.
To answer the basic question we investigate the expression and intracellular localization of septin isoforms, and their connection with other proteins in myogenic C2C12 culture and skeletal muscles originated from mice. Molecular biological, classical and fluorescent histological methods are used (mild muscle injury induced by BaCl injection, isolation of RNA- and protein samples, preparation of skeletal muscles from different regions and single muscle fibers by enzymatic digestion, RT-PCR, qPCR, western blot, immuncytochemistry, immunhistochemistry, confocal microscopy, immunprecipitation, mass spectrometry).
- Is the regeneration process modified in Septin 7 KD mice?
To study the role of Septin 7 we established Septin 7 partial knockdown (KD) mice by using Cre/Lox system and C2C12 cultures with decreased expression of Septin7 by shRNA-based gene silencing. Alteration in Septin7 KD cultures we apply cell viability, cell proliferation assays, differentiation is also followed, while we measure in vivo and in vitro force generation in mice (voluntary running, Grip test, force measurement on isolated EDL and Soleus muscles ). Molecular biological (genotyping, RNA- and protein isolation RT-PCR, qPCR, western blot), classical histochemistry techniques are used to determine morphological, structural and functional changes generated by Septin7 modification (preparation of skeletal muscles and single muscle fibers, immuncytochemistry, immunhistochemistry, confocal microscopy, mitochondrial labelling, electron microscopy (TEM, SEM), functional measurements (detection of mitochondrial membrane potential alterations, intracellular calcium measurements, electrophysiology, fatigue protocols under Live confocal microscopy).
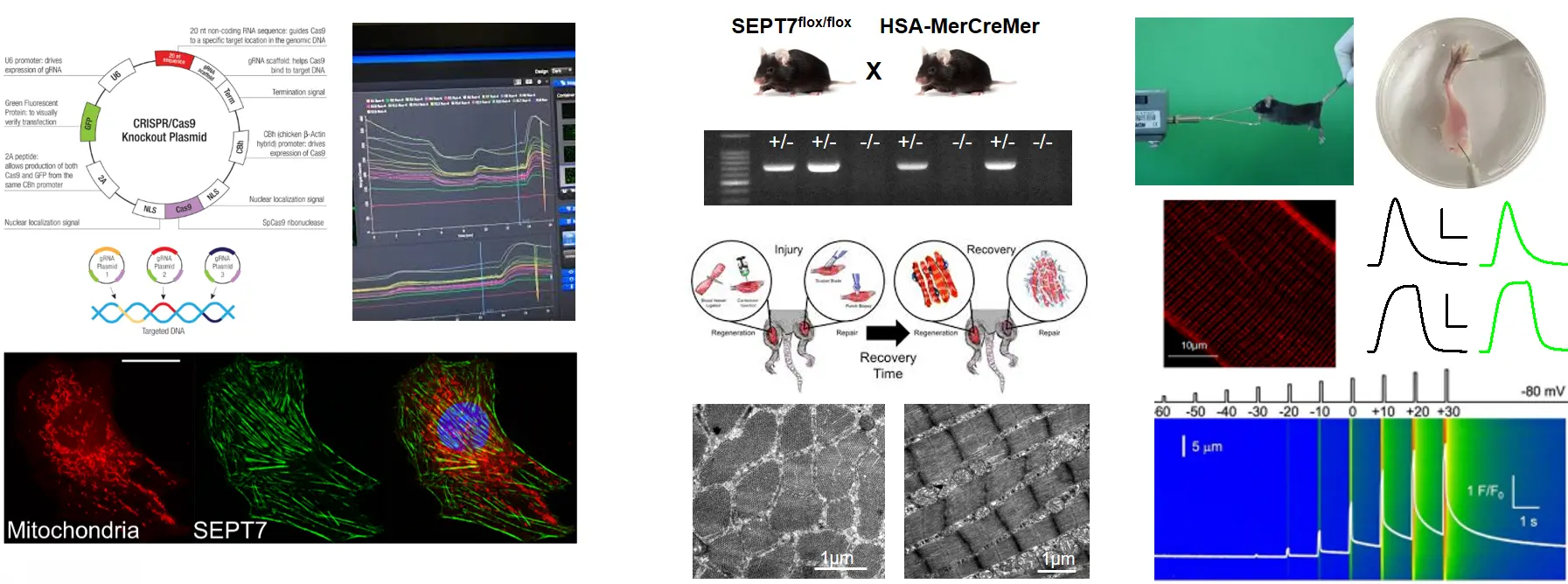
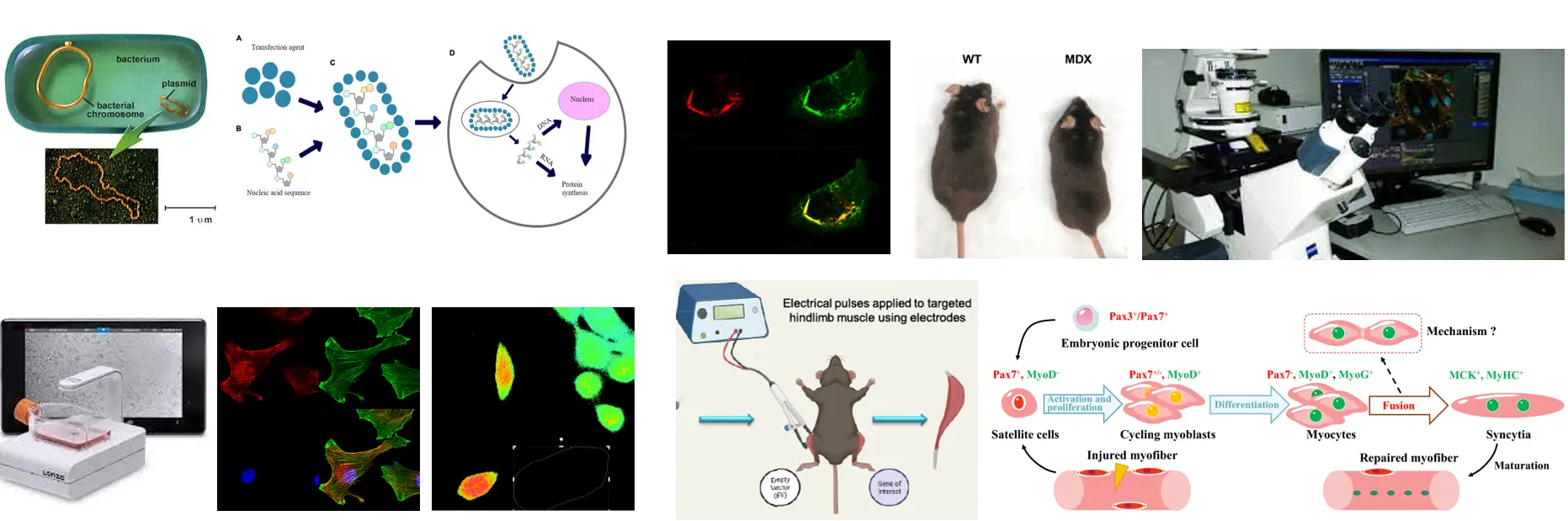
- Does Septin 7 expression show any difference in MDX mice (the most commonly used mouse model for Duchenne muscular dystrophy)?
The previous measurements will be repeated in a model system of muscular dystrophy. We also investigate how fluorescently tagged Septin7 expression modify the structure and function of skeletal muscles. To answer this we use bacterial transformation and plasmid isolation, lipid-mediated transfcetion and electroporation.
In cell cultures we examine the effect of Septin7 gene silencing to cell migration, and also the expression of fluorescently tagged Septin7 using confocal microscopy, Cytosmart and OperaPhenyx systems. Understanding the function of Septin 7 in skeletal muscle regeneration can get access to better therapeutic approaches in treating muscle injuries and disorders accompanied by pathological muscle regeneration.
- Publications related to the project:
Gönczi, M., Dienes, B., Dobrosi, N., Fodor, J., Balogh, N., Oláh, T., Csernoch, L.: Septins, a cytoskeletal protein family, with emerging role in striated muscle. J. Muscle Res. Cell Motil. 42 (2), 251-265, 2021. https://doi.org/10.1007/s10974-020-09573-8
Gönczi, M., Ráduly, Z., Szabó, L., Fodor, J., Telek, A., Dobrosi, N., Balogh, N., Szentesi, P., Kis, G., Antal, M., Trencsényi, G., Dienes, B., Csernoch, L.: Septin7 is indispensable for proper skeletal muscle architecture and function. eLife. 11 e75863, 2022. https://doi.org/10.7554/eLife.75863
- Conference abstracts related to the project:
Telek, A., Fodor, J., Dobrosi, N., Szabó, L., Gönczi, M., Dienes, B., Csernoch, L.: Septin-7 is indispensable in skeletal muscle regeneration.J. Gen. Physiol. 154 (9), 1, 2022. https://doi.org/10.1085/jgp.2021ecc33
Gönczi, M., Fodor, J., Telek, A., Dobrosi, N., Ráduly, Z., Szabó, L., Szentesi, P., Dienes, B., Csernoch, L.: The Role of Septin7 in Skeletal Muscle Regeneration. Biophys. J. 120 (3), 96a, 2021. https://doi.org/10.1016/j.bpj.2020.11.792
Csernoch, L., Gönczi, M., Ráduly, Z., Szabó, L., Dobrosi, N., Szentesi, P., Dienes, B.: Essential Role of Septin 7 in Skeletal Muscle Structure and Function. Biophys. J. 118 (Suppl.), 258a, 2020. https://doi.org/10.1016/j.bpj.2019.11.1500
Gönczi, M., Szabó, L., Ráduly, Z., Dobrosi, N., Kis, G., Cseri, K., Dienes, B., Csernoch, L.: Mitochondrial Organization is Severly Modified in Skeletal Muscles of Septin 7 Knockdown Animals. Biophys. J. 118 (Suppl.), 277a, 2020. https://doi.org/10.1016/j.bpj.2019.11.1589
Angyal, Á., Gönczi, M., Ráduly, Z., Szabó, L., Dobrosi, N., Dienes, B., Csernoch, L.: Septin 7 has an essential role in differentiation of C2C12 cells.J. Muscle Res. Cell Motil. 40 (2), 242-243, 2019. http://hdl.handle.net/2437/322063
Dobrosi, N., Szabó, L., Ráduly, Z., Gönczi, M., Kis, G., Cseri, K., Dienes, B., Csernoch, L.: Septin 7 has no role in ec-coupling but severly modifies skeletal muscle architecture. J. Muscle Res. Cell Motil. 40 (2), 250, 2019. http://hdl.handle.net/2437/322060